Safety considerations
Discussing the potential risks of TYSABRI
Every person is different and may react to TYSABRI differently. Just as there are potential benefits with TYSABRI, there are also potential risks. It is important to talk with your healthcare team about both.
JC Virus and PML
TYSABRI increases your risk of getting a rare brain infection—called progressive multifocal leukoencephalopathy (PML)—that usually leads to death or severe disability.
- You can only get PML if you’ve been infected with the JC Virus.
- Your risk of PML is less than 1% regardless of JC Virus status.
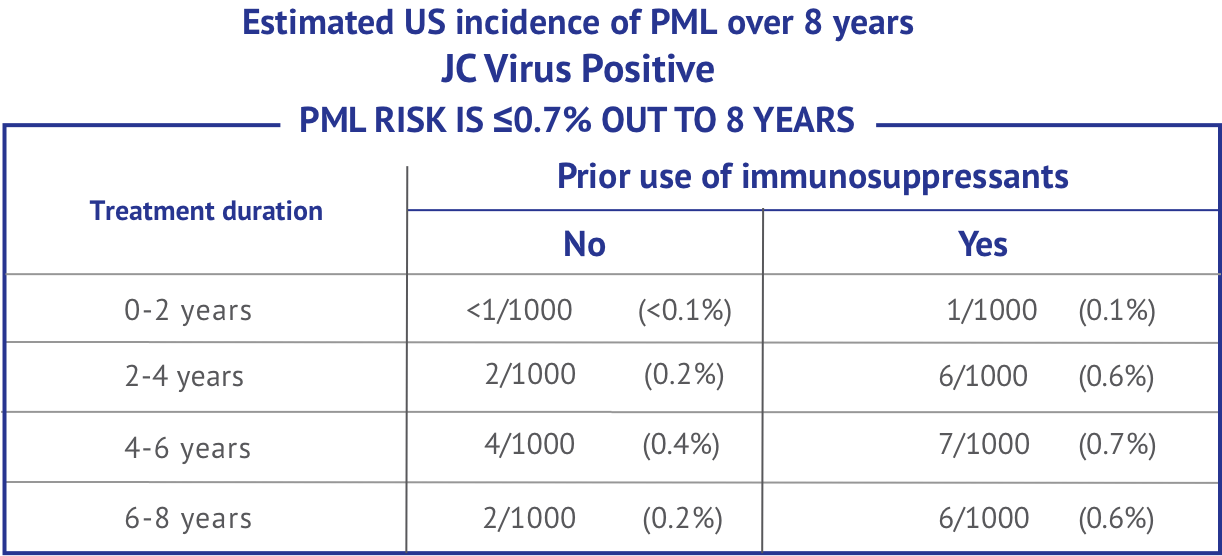
Today, there are 8 years of available data to help understand the estimated risk of PML while taking TYSABRI. Your doctor can help you understand your risk and make the right treatment decision for you. Read the questions below to find out more.
Virus and PML?
Approximately 50%-60% of the general public is positive for the John Cunningham (JC) Virus. It's that common, and usually, it is harmless. However, the JC Virus can cause PML in people with weakened immune systems.
PML is a rare brain infection that usually leads to death or severe disability. It is a possible side effect of taking TYSABRI. The estimated risk of developing PML is less than 1%. As of today, there is no treatment, prevention, or cure for PML.
You can only develop PML if you’ve been infected by the JC Virus. It is important to know that testing positive for JC Virus antibodies does not mean that you will get PML. You can continue being treated with TYSABRI even if you are JC Virus positive. It is something you will want to discuss with your doctor and healthcare team, and get their recommendation.
Your risk for PML is higher if you:
- are positive for antibodies to the JC Virus
- have received certain medications that can weaken your immune system before you start receiving TYSABRI
- have received TYSABRI for a long time
Your risk of getting PML is greatest if you have all 3 risk factors listed above. There may be other risk factors that have not yet been identified. You can continue being treated with TYSABRI even if you are JC Virus positive.


to know about
testing
for the
JC Virus?
As long as you are taking TYSABRI, you and your healthcare provider will evaluate your risks and monitor any changes to your health.
Your healthcare provider will periodically retest you for the JC Virus antibodies so you can make the best treatment decision, together.
of PML?
Based on data from 100,000 US patients over 8 years, it is estimated that there is less than or equal to 0.7% risk to develop PML.
The rate of PML among people who are negative for JC Virus antibodies is 1 in 10,000—or 0.01%—regardless of other risk factors.

However, it is still recommended that you continue to be tested regularly over the course of your TYSABRI treatment. It is still possible, but not common, to have a false negative test result or become JC Virus antibody positive. The reported rate of people with relapsing MS who change from JC Virus antibody negative to antibody positive is 3% to 8% annually.
Biogen offers a free blood test (known as Stratify JCV™ antibody).
TOUCH
Prescribing
Program help
me?
With the TOUCH® Prescribing Program, you and your healthcare team monitor your risk over time and decide whether or not TYSABRI remains right for you.
The TOUCH program includes your:
- doctor
- nurse
- pharmacy
- infusion center
The program is designed for your safety, and is available for as long as you are treated with TYSABRI.
Biogen offers a free blood test (known as Stratify JCV™ antibody).
Serious side effects
TYSABRI can cause serious side effects. As with any treatment, being aware of how it may affect you is important. If you have any of the symptoms listed below, call your healthcare professional right away:
- Herpes infections, encephalitis, or meningitis. Symptoms include sudden fever, severe headache, or confusion. Infection of the eye caused by herpes viruses leading to blindness in some patients has occurred. Call your healthcare professional if you have changes in vision, redness, or eye pain
- Liver damage. Symptoms include yellowing of the skin and eyes (jaundice), unusual darkening of the urine, nausea, feeling tired or weak, or vomiting
- Allergic reactions (eg, hives, itching, trouble breathing, chest pain, dizziness, wheezing, chills, rash, nausea, flushing of skin, low blood pressure), including serious allergic reactions (eg, anaphylaxis). Serious allergic reactions usually happen within 2 hours of the start of the treatment, but they can happen any time after receiving TYSABRI
- Weakened immune system. TYSABRI may increase your risk of getting an unusual or serious infection
- Low platelet counts. Symptoms include easy bruising, small scattered spots on your skin that are red, pink or purple, heavier menstrual periods than are normal, bleeding from your gums or nose that is new or takes longer than usual to stop, or bleeding from a cut that is hard to stop
Other possible side effects
What other side effects should I be aware of?
In addition to the serious side effects, TYSABRI can cause other side effects as well, including:
- headache
- urinary tract infection
- lung infection
- pain in your arms and legs
- vaginitis
- stomach-area pain
- feeling tired
- joint pain
- depression
- diarrhea
- rash
- nose and throat infections
- nausea
If you experience any side effect that bothers you or does not go away, tell your healthcare provider.
What else should I tell my healthcare provider?
Before receiving TYSABRI, it is important to tell your healthcare provider:
- If you have a medical condition that can weaken your immune system, such as:
- HIV infection or AIDS
- leukemia or lymphoma
- organ transplant
- If you are pregnant or plan to become pregnant. TYSABRI may cause low platelets, and in some cases also low red blood cells (anemia), in your newborn baby if you take TYSABRI while you are pregnant. It is not known if TYSABRI can cause birth defects
- If you are breastfeeding or plan to breastfeed. TYSABRI can pass into your breast milk. It is not known if TYSABRI that passes into your breast milk can harm your baby. Talk to your doctor about the best way to feed your baby while you receive TYSABRI
- About all the medicines and supplements you take, especially medicines that can weaken your immune system. If you are not sure, ask your doctor
You may be treated with TYSABRI for as long as you and your healthcare team decide it is right for you. In the US, more than 50% of people being treated with TYSABRI have been using it for more than 5 years.*
*Of the 23,190 patients taking TYSABRI, 12,803 (55.2%) have been treated with TYSABRI for >5 years as of March 2025. TYSABRI was studied in a 2-year clinical trial, and your risk of developing PML can increase over time. Talk with your doctor to ensure the benefits continue to outweigh the risks.